Tissue Transplants Utilizing Extracellular Matrix Preservation (BioECM) Technology
CarePath Biologics commercializes and is further developing BioECM allografts for transplantation of age zero tissues into a recipient’s mature tissues while preserving the functionality of human placenta grafts.
CarePath Biologics is a complete vertically integrated regenerative medicine company focused on the development, manufacture, and commercialization of product solutions for the Advanced Wound Care market, with future products for Surgical & Sports Medicine markets, like no other in this space.
Current market tissue products are processed through our sister company Human Regenerative Technologies, which is an AATB licensed tissue bank.
Through that division, we are processing industry leading unique tissue transplants that are shelf stable, preserving integrity while maintaining their natural properties. Traditionally, procedures have used biologically passive approaches to repair injured tissues by removing, re-attaching or stabilizing damaged, severed, fractured or problematic tissues with sutures, stabilization or fixation devices. Common post-op complications and challenges are due to variables beyond their control, including the severity of the injury, co-morbidities, patient biology, compliance and other factors, and the body is unable to heal properly to its original state.
CarePath Biologics BioECM® preservation utilizes the richest natural intact allograft tissue known to exist. With our novel HydraTek® technology, we can maximize preservation and potential through our unique preservation system, to further advance medical treatments. Skye® products provide distinct tissue and safety advantages over traditional human allograft or xenograft tissue sources. Placental tissues provide the most abundant source of an extracellular matrix (ECM).
Tissue Transplants for Unique Clinical Challenges
Complex clinical challenges demand a wide range of solutions.
At Skye, we are continually perfecting our BioECM® transplant preservation to maximize utility across many clinical specialties and procedures.
HydraTek Innovation
CarePath Biologics commercializes and is further developing BioECM allografts for transplantation of age zero tissues into a recipient’s mature tissues while preserving the functionality of human placenta grafts.
Unsurpassed Handling & Delivery
As a result, HydraTek® is able to deliver unique easy to handle tissues exceeding industry standards and commercially available options.
The Preserved Source
The Utmost in Safety
HydraTek® provides an outstanding standard of safety through its stringent donor screening, testing, and strict quality control, providing stable and sterile native tissues. All testing and safety standards meet or exceed the FDA requirements.
Unique Allograft Delivery Forms
Unsurpassed Handling & Delivery
HydraTek® is the only process designed to harvest and preserve The Complete Placental Connective Tissue Matrix® from age zero placental tissues in an ideal native tissue allograft transplant form with the recipient in mind, giving doctors a very ready to use allograft that is easy to handle and apply.
Industry First: Room Temperature Connective Tissue Transplant
Excellerate® Technology
BioAware® Membrane Preservation System
FastActing® Membrane Performance
Unique Allograft Delivery Forms
Placental tissues have been shown in the literature to be naturally anti-inflammatory, anti-fibrotic, anti-microbial, to modulate inflammation, promote tissue remodeling and reduce scar tissue formation in clinical applications. They have been used medically for decades and one reason they are an excellent transplant is they are also an immunoprivileged tissue type. They have been shown to not cause a host rejection.
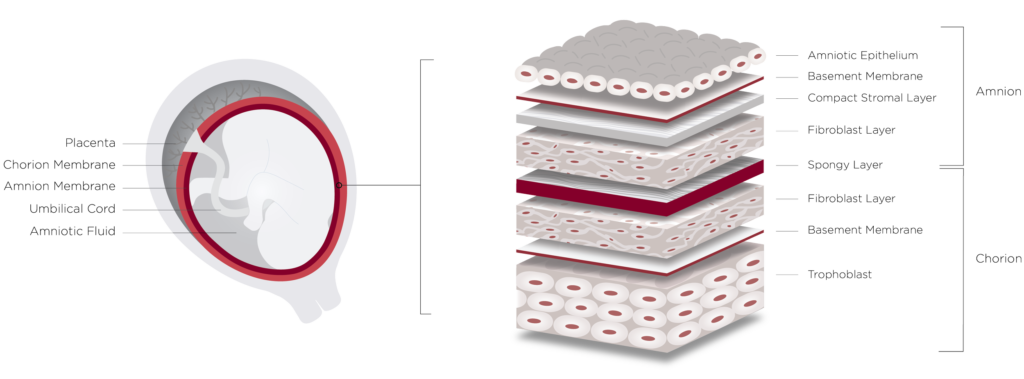
Since the early 1900’s, amniotic membranes have been used clinically as wound coverings to treat chronic and acute wounds. Over the last few decades, tissue banks have made some basic membrane products commercially available, derived from individual amniotic membranes and produced using traditional processing methods.
These preliminary membrane products are developed specifically to treat topical wound applications. However, more significant injuries, degeneration, and surgical conditions require richer biologic solutions and improved product and delivery forms. Advanced transplants demand new processing technology, as traditional processing is limited by older methods and not designed to effectively process and preserve these unique age zero tissue transplants in their native state.
Adhering to strict safety standards, HydraTek® obtains its source tissues from healthy mothers who are under the care of a licensed OB/GYN physician at a partner facility. All donations are sourced from U.S.-based donors, and only after live, healthy cesarean birth delivery. No maternal or fetal tissues are collected during the process. Intensive safety testing is used to screen both the donor and donated tissues before processing.
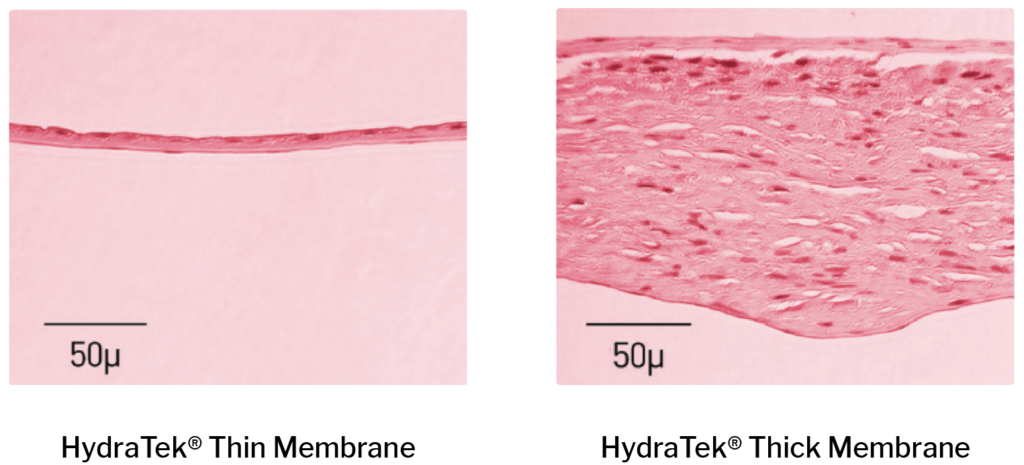
Improved Tissue Integrity
HydraTek® Delivers Proven Tissue Transplant Potential.
*comparison data on file